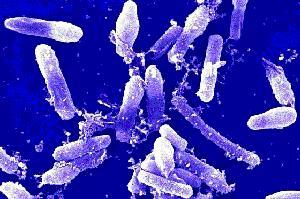
A review of warning letters U.S. Food and Drug Administration indicates that contamination events associated with Bacillus species represents a relatively high proportion of microbiological related citations. During the period March 2013 to August 2014, 7 warning letters relating to contamination from spore-forming bacteria were issued. Extending the review back to 2007, it is noteworthy that the most common microorganism associated with contamination is Bacillus cereus.
This article considers some of the inspectorate findings relating to Bacillus contamination and goes on to consider the implications for the control of pharmaceutical products manufacture that arise from these regulatory observations. The discussion has a focus on Bacillus cereus, given its relative ubiquity, and extends to the general risks that arise from spore-forming microorganisms and the risk-mitigating actions that can be taken.
This is the basis of a new article by Tim Sandle for American Pharmaceutical Review. The article is available to be viewed in full on-line. Go to: APR.


No comments:
Post a Comment
Pharmaceutical Microbiology Resources