Over
the last few years, research on bacteria in the digestive tract (gut microbiome) has
confirmed the major role they play in our health. An international consortium
has developed the most complete database of microbial genes ever created. The
catalogue features nearly ten million genes and will constitute a reference for
all research on gut bacteria.
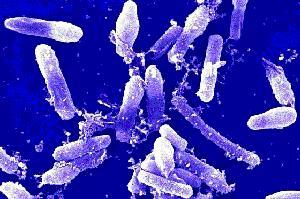
Research
on the gut microbiome (all of the bacteria in the digestive tract) has
multiplied over the past several years, helped in great part by new sequencing
technologies. The gut microbiome, which scientists have labelled a "new
organ" that is composed of tens of trillions of bacteria -- ten times as
many as the number of cells in the human body -- is directly linked to the
immune system and brain. It is a major player in chronic illnesses such as
obesity and Type 2 diabetes. However, research in the field depends on access
to reference gene databases (or catalogues), which is particularly important
when identifying the functions of microbial genes. Few and far between,
existing catalogues were created using samples from a limited number of people
and geographical origins.
INRA
researchers, as part of the MetaHIT international consortium, put together a
catalogue of microbial genes that regroups pre-existing catalogues (European,
American and Chinese) and enhanced it with new sequences. Apart from being an
unparalleled resource, the analyses done for the catalogue showed that it
contains the broadest collection of microbial genes -- and by extension, their
functions -- present in the global population. With ten million genes, this new
catalogue presents the most impressive array of human intestinal bacteria in
the world.
Most
of the genes (around six million) are shared by just 1% of the population,
making them quite rare. While there is substantial data today regarding the
most common genes, future research will focus on determining the importance and
role of these rare genes.
Thanks
to this catalogue, the most clinically significant genes can be described, most
notably those related to illnesses such as Type 2 diabetes, cirrhosis of the
liver, cardiovascular diseases and some cancers. It will also provide a more
complete picture of imbalances in the gut microbiome (dysbiosis), particularly
those caused by medication.
For
further details see:
An integrated catalog of reference genes in the human gut
microbiome. Nature Biotechnology, 2014; 32 (8): 834 DOI: 10.1038/nbt.2942
Posted by Tim Sandle





 Ms. Sharp has been the chief financial officer and senior vice president, finance, of Ultragenyx, a clinical-stage biotechnology company developing medicines to treat rare diseases, since 2012. She is a member of the Board of Directors of Agenus Inc. (formerly Antigenics Inc.), a publicly traded biotechnology firm, where she served as chief financial officer from 2006 to 2012. Prior to Agenus, Ms. Sharp held strategic planning and corporate finance roles at Elan Pharmaceuticals. She has also worked with McKinsey & Company and Goldman Sachs, specializing in pharmaceuticals and medical devices. Ms. Sharp holds both a BA and an MBA from Harvard University.
Ms. Sharp has been the chief financial officer and senior vice president, finance, of Ultragenyx, a clinical-stage biotechnology company developing medicines to treat rare diseases, since 2012. She is a member of the Board of Directors of Agenus Inc. (formerly Antigenics Inc.), a publicly traded biotechnology firm, where she served as chief financial officer from 2006 to 2012. Prior to Agenus, Ms. Sharp held strategic planning and corporate finance roles at Elan Pharmaceuticals. She has also worked with McKinsey & Company and Goldman Sachs, specializing in pharmaceuticals and medical devices. Ms. Sharp holds both a BA and an MBA from Harvard University.